Hidrogeles en el Tratamiento de la Tendinopatía Degenerativa
DOI:
https://doi.org/10.17488/RMIB.44.3.3Palabras clave:
hidrogel, liberación de fármaco, tendinopatíaResumen
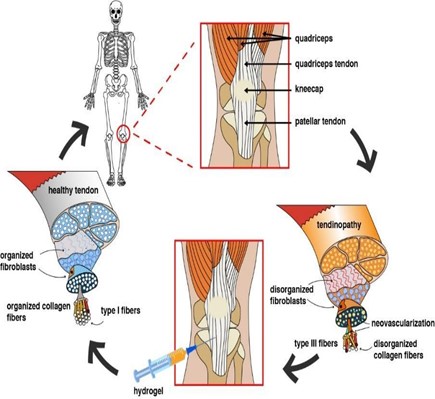
La tendinopatía degenerativa es un importante problema de salud, y su incidencia aumenta cada año en todo el mundo. Esta condición genera déficits funcionales en pacientes jóvenes o adultos, así como en personas sedentarias o activas, trayendo consigo repercusiones sanitarias, sociales y económicas. Debido al suministro de sangre limitado, la administración de medicamentos es compleja para las enfermedades de los tendones, como la tendinopatía degenerativa. El uso de biomateriales, como los hidrogeles, ha ganado una atención significativa en el diseño de sistemas de administración de fármacos para tratar patologías musculoesqueléticas debido a sus atractivas características y los desafíos que plantean las rutas convencionales de administración de fármacos. Este documento proporciona una descripción general de la patología del tendón y analiza el uso de hidrogeles como transportadores de fármacos y agentes de liberación en tratamientos emergentes.
Descargas
Citas
W.L. Lim, L.L. Liau, M.H. Ng, S.R. Chowdhury, and J.X. Law J. X., “Current progress in tendon and ligament tissue engineering”, J. Tissue Eng. and Regen. Med., vol. 16, no. 6, 2019, 549-571, doi: 10.1007/s13770-019-00196-w
C. Loiacono, S. Palermi, B. Massa, I. Belviso, V. Romano, A. Di Gregorio, A.M. Sacco, “Tendinopathy: Pathophysiology, therapeutic options, and role of nutraceutics. A narrative literature review”, Medicina, vol. 55, no. 8, 2019, 447, doi: 10.3390/medicina55080447
L. Subervier Ortiz, B. Jaramillo Loranca, M. Villanueva Ibáñez, “La tendinopatía degenerativa y su abordaje multidisciplinario desde la evidencia científica”, Ciencias multidisciplinarias Proceedings T-IV Ecorfan, vol. 4, 2020, 1-18.
R.L. Martin, R. Chimenti, T. Cuddeford, J. Houck, J.W. Matheson, C.M. McDonough, S. Paulseth, D.K. Wukich, C.R. Carcia, “Achilles Pain, Stiffness, and Muscle Power Deficits: Midportion Achilles Tendinopathy Revision”, J. Orthop. Sports Phys. Ther., vol. 48, no. 5, 2018, 2-11, doi:10.2519/jospt.2010.0305
P. Malliaras, “Physiotherapy management of Achilles tendinopathy”, J. Physiother., vol. 68, no. 4, 221-237, 2022, doi: 10.1016/j.jphys.2022.09.010
B. Homayun, X. Lin, and H.J. Choi, “Challenges and recent progress in oral drug delivery systems for biopharmaceuticals”, Pharmaceutics, vol. 11, no.3, 2019, 129, doi: 10.3390/pharmaceutics11030129
J. Li, D.J. Mooney D. J., “Designing hydrogels for controlled drug delivery”, Nat. Rev. Mater., vol.1, no. 12, 2016, 1-17, doi: 10.1038/natrevmats.2016.71
J. Bojsen-Møller, S.P. Magnusson, “Mechanical properties, physiological behavior, and function of aponeurosis and tendon”, J. Appl. Physiol., vol. 126, no. 6, 2019, 1800-1807, doi: 10.1152/japplphysiol.00671.2018
R. Aicale, A. Oliviero, and N. Maffulli, “Management of Achilles and patellar tendinopathy: what we know, what we can do”, J. foot. Ankle. Res, vol.13, no. 1, 2020, 1-10, doi: 10.1186/s13047-020-00418-8
D. King, G. Yakubek, M. Chughtai, A. Khlopas, P. Saluan, M.A. Mont, and J. Genin, “Quadriceps tendinopathy: a review part 1: epidemiology and diagnosis”, Ann. Transl. Med., vol. 7, no. 4, 2019, doi: 10.21037/atm.2019.01.58
N.L. Millar, K.G. Silbernagel, K. Thorborg, P.D. Kirwan, L.M. Galatz, G.D. Abrams, S.A. Rodeo, “Tendinopathy”, Nat. Rev. Dis. Primers, vol. 7, no.1, 2021, 1-21, doi: 10.1038/s41572-020-00234-1
S. Crimaldi, S. Liguori, P. Tamburrino, A. Moretti, M. Paoletta, G. Toro, and G. Iolascon, “The role of hyaluronic acid in sport-related tendinopathies: a narrative review”, Medicina, vol.57, no. 10, 2021; 1088, doi: 10.3390/medicina57101088
B.J. Dean, S.G. Dakin, N.L. Millar, A.J. Carr, “Emerging concepts in the pathogenesis of tendinopathy”, The Surgeon, vol. 15, no. 6, 2017, 349-354, doi: 10.1016/j.surge.2017.05.005
H.S. Semis, C. Gur, M. Ileriturk, F.M. Kandemir, O. Kaynar, “Evaluation of therapeutic effects of quercetin against Achilles tendinopathy in rats via oxidative stress, inflammation, apoptosis, autophagy, and metalloproteinases”, Am. J. Sports Med., vol. 50, no.2, 2022, 486-498, doi: 10.1177/03635465211059821
L. Gaut, and D. Duprez, “Tendon development and diseases”, Wiley Interdiscip. Rev., Dev. Biol., vol. 5, no. 1, 2016, 5–23, doi: 10.1002/wdev.201
D. Singh, S.K. Srivastava, T.K. Chaudhuri, G. Upadhyay, “Multifaceted role of matrix metalloproteinases (MMPs)”, Front. Mol. Biosci., vol. 2, 2015, 19, doi: 10.3389/fmolb.2015.00019
A. D'Addona, N. Maffulli, S. Formisano, D. Rosa, “Inflammation in tendinopathy”, The Surgeon, vol. 15, no. 5, 2017, 297-302, doi: 10.1016/j.surge.2017.04.004
J. Maquirriain, A. Kokalj, “Management of acute Achilles tendinopathy: effect of etoricoxib on pain control and leg stiffness”, Georgian Med. News, no. 222, 2013, 36–43.
H.Y. Li, and Y.H. Hua, “Achilles tendinopathy: current concepts about the basic science and clinical treatments”, Biomed. Res. Int., vol. 2016, 2016; doi: 10.1155/2016/6492597
A. Bittermann, S. Gao, S. Rezvani, J. Li, K.J. Sikes, J. Sandy, A. Plaas, “Oral ibuprofen interferes with cellular healing responses in a murine model of Achilles tendinopathy” J. musculoskelet. Disord. Treat., vol. 4, no. 2, doi:10.23937/2572-3243.1510049
W. Liu, Y. Cao, Biomaterials for engineered tendon regeneration, in Biomaterials and Regenerative Medicine Editor: Peter X. Ma, University of Michigan, Ann Arbor. Cambridge University Press (CUP), Cambridge: 478–487.
F. Abat, H. Alfredson, M. Cucchiarini, H. Madry, A. Marmotti, C. Mouton, and L. de Girolamo, “Current trends in tendinopathy: consensus of the ESSKA basic science committee. Part II: treatment options”, J. Exp. Orthop. vol. 5, 2018, 1-17, doi: 10.1186/s40634-018-0145-5
J.H. Lee, and H.W. Kim, “Emerging properties of hydrogels in tissue engineering”, J. Tissue Eng., vol. 9, 2041731418768285, doi: 10.1177/2041731418768285
Y. Zhang, Z. Li, J. Guan, Y. Mao, P. Zhou, “Hydrogel: A potential therapeutic material for bone tissue engineering”, AIP Adv., vol. 11, no. 1, 2021, 010701, doi:10.1063/5.0035504
S. Deepthi, M.N. Sundaram, J.D. Kadavan, R. Jayakumar, “Layered chitosan-collagen hydrogel/aligned PLLA nanofiber construct for flexor tendon regeneration”, Carbohydr. Polym., vol. 153, 2017, 492-500, doi: 10.1039/c7tb00601b
A. Elosegui-Artola, “The extracellular matrix viscoelasticity as a regulator of cell and tissue dynamics”, Curr. Opin. Cell Biol., vol. 72, 2021, 10-18, doi: 10.1016/j.ceb.2021.04.002
C.F. Guimarães, L. Gasperini, A.P. Marques, and R.L. Reis, “The stiffness of living tissues and its implications for tissue engineering”, Nat. Rev. Mater., vol. 5, no. 5, 2020, 351-370, doi: 10.1038/s41578-019-0169-1
S.S. Gupta, A. Meena, T. Parikh, A. T. Serajuddin, “Investigation of thermal and viscoelastic properties of polymers relevant to hot melt extrusion-I: Polyvinylpyrrolidone and related polymers”, J. Excip. Food Chem., vol. 5, no. 1, 2016, 32-45.
O. Chaudhuri, L. Gu, D. Klumpers, M. Darnell, S.A. Bencherif, J.C. Weaver, and D.J. Mooney, “Hydrogels with tunable stress relaxation regulate stem cell fate and activity”, Nat. Mater., vol. 15, no. 3, 2016, 326-334, doi: 10.1038/nmat4489
B. Toprakhisar, A. Nadernezhad, E. Bakirci, N. Khani, G.A. Skvortsov, B. Koc, “Development of bioink from decellularized tendon extracellular matrix for 3D bioprinting”, Macromol. Biosci., vol. 18, no. 10, 2018, 1800024, doi: 10.1002/mabi.201800024
Y. Ma, T. Han, Q. Yang, J. Wang, B. Feng, Y. Jia, and F. Xu, “Viscoelastic Cell Microenvironment: Hydrogel‐Based Strategy for Recapitulating Dynamic ECM Mechanics”, Adv. Funct. Mater., vol. 31, no. 24, 2021, 2100848, doi: 10.1002/adfm.202100848
E.E Antoine, P.P. Vlachos, and M.N. Rylander, “Review of collagen I hydrogels for bioengineered tissue microenvironments: characterization of mechanics, structure, and transport” Tissue Eng. Part B: Rev, vol. 20, no. 6, 2014, 683-696, doi: 10.1089/ten.teb.2014.0086
P.Y. Chou, S.H. Chen, C.H. Chen, S.H. Chen, Y.T. Fong, J.P. Chen, “Thermo-responsive in-situ forming hydrogels as barriers to prevent post-operative peritendinous adhesion”, Acta Biomater., no. 63, 2017, 85-95, doi: 10.1016/j.actbio.2017.09.010
M. Bartoš, T. Suchý, and R. Foltán, “Note on the use of different approaches to determine the pore sizes of tissue engineering scaffolds: ¿what do we measure?”, Biomed. Eng. Online, vol. 17, no. 1, 2018, 1-15, doi: 10.1186/s12938-018-0543-z
H. Nasution, H. Harahap, N.F. Dalimunthe, M.H.S. Ginting, M. Jaafar, O.O. Tan, A.L. Herfananda, “Hydrogel and effects of crosslinking agent on cellulose-based hydrogels: A review”, Gels, vol. 8, no. 9, 2022, 568, doi: 10.3390/gels8090568
L. Wang, M. Neumann, T. Fu, W. Li, X. Cheng, B.L. Su, “Porous and responsive hydrogels for cell therapy”, Curr. Opin. Colloid Interface Sci., vol. 38, 2018, 135-157, doi: https://doi.org/10.1016/j.cocis.2018.10.010
S. Garg, A. Garg, R.D. Vishwavidyalaya, “Hydrogel: Classification, properties, preparation and technical features”, Asian J. Biomater. Res, vol. 2, no. 6, 2016, 163-170.
S.H. Aswathy, U. Narendrakumar, I. Manjubala, “Commercial hydrogels for biomedical applications” Heliyon, vol. 6, no. 4, 2020, e03719, doi: 10.1016/j.heliyon.2020.e03719
D. Sandrin, D. Wagner, C.E. Sitta, R. Thoma, S. Felekyan, H.E. Hermes, C.A. Seidel, “Diffusion of macromolecules in a polymer hydrogel: from microscopic to macroscopic scales”, Phys. Chem. Chem. Phys., vol. 18, no. 18, 2016, 12860-12876, doi:10.1039/c5cp07781h
E. Axpe, D. Chan, G.S. Offeddu, Y. Chang, D. Merida, H.L. Hernandez, E.A. Appel, “A multiscale model for solute diffusion in hydrogels”, Macromolecules, vol. 52, no. 18, 2019, 6889-6897, doi: 10.1021/acs.macromol.9b00753
M. Stoppato, F.V. Flotow and C.K. Kuo, Application of Hydrogels for Tendon and Ligament Repair and Tissue Engineering. In GELS HANDBOOK: Fundamentals, Properties and Applications Vol. 2: Applications of Hydrogels in Regenerative Medicine, 2016, 271-293.
M.S. Rehmann and A.M. Kloxin, “Tunable and dynamic soft materials for three-dimensional cell culture”, Soft matter., vol. 9, no.29, 2013, 6737-6746, doi: 10.1039/C3SM50217A
X. Guan, M. Avci‐Adali, E. Alarçin, H. Cheng, S.S. Kashaf, Y. Li, and A. Khademhosseini, “Development of hydrogels for regenerative engineering”, Biotechnol. J, vol. 12, no. 5, 2017, 1600394, doi:10.1002/biot.201600394
B. Ozcelik, Degradable hydrogel systems for biomedical applications, I Biosynthetic Polymers for Medical Applications, 2016, 173-188, Woodhead Publishing.
E.D. Silva, P.S. Babo, R. Costa-Almeida, R.M. Domingues, B.B. Mendes, E. Paz, M.E. Gomes, “Multifunctional magnetic-responsive hydrogels to engineer tendon-to-bone interface”, Nanomed. Nanotechnol. Biol. Med., vol. 14, no. 7, 2018, 2375-2385, doi: 10.1016/j.nano.2017.06.002
T. Kirchgesner, A. Larbi, P. Omoumi, J. Malghem, N. Zamali, J. Manelfe, B. Dallaudière, “Drug-induced tendinopathy: from physiology to clinical applications”, Joint Bone Spine, vol. 81, no.6, 2014, 485-492. 10.1016/j.jbspin.2014.03.022
S. Mansoor, P.P. Kondiah, Y.E. Choonara “Advanced hydrogels for the controlled delivery of insulin”, Pharmaceutics, vol. 13, no. 12, 2021, 2113, doi: 10.3390/pharmaceutics13122113
Y. Dong, W. Wang, O. Veiseh, E.A. Appel, K. Xue, M.J. Webber and D.G. Anderson, “Injectable and Glucose-Responsive Hydrogels Based on Boronic Acid–Glucose Complexation”, Langmuir, vol. 32, no. 34, 2016, 8743–8747, doi: 10.1021/acs.langmuir.5b04755
O. Prucker, T. Brandstetter, J. Rühe, “Surface-attached hydrogel coatings via C, H-insertion crosslinking for biomedical and bioanalytical applications”, Biointerphases, vol. 13, no. 1, 2018, 010801, doi: 10.1116/1.4999786
Y.L. Zhou, Q.Q. Yang, Y.Y. Yan, C. Zhu, L. Zhang, J.B. Tang, “Localized delivery of miRNAs targets cyclooxygenases and reduces flexor tendon adhesions”, Acta Biomater., vol. 70, 2018, 237-248, doi: 10.1016/j.actbio.2018.01.047
E. Caló, V.V. Khutoryanskiy, “Biomedical applications of hydrogels: A review of patents and commercial products”, Eur. Polym. J., vol. 65, 2015, 252-267, doi: 10.1016/j.eurpolymj.2014.11.024
P. Mather, J. Wu, D. Ren, S. Hou, US Patent 8,431,151 B2; 2013
L.E. Kass, J. Nguyen, “Nanocarrier‐hydrogel composite delivery systems for precision drug release”, Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol., vol. 14, no. 2, 2021, e1756, doi: 10.1002/wnan.1756
Y. Zhao, B. Ran, X. Xie, W. Gu, X. Ye, J. Liao, “Developments on the Smart Hydrogel-Based Drug Delivery System for Oral Tumor Therapy”, Gels, vol. 8, no. 11, 2022, 741, doi: 0.3390/gels8110741
L.A. Sharpe, A.M. Daily, S.D. Horava, N.A. Peppas, “Therapeutic applications of hydrogels in oral drug delivery”, Expert opin. drug deliv., vol. 11, no. 6, 2014, 901-915, doi: 10.1517/17425247.2014.902047
N.S. Malik, M. Ahmad, M.U. Minhas, “Cross-linked β-cyclodextrin and carboxymethyl cellulose hydrogels for controlled drug delivery of acyclovir”, PloS one, vol.12, no. 2, 2017, e0172727, doi: 10.1371/journal.pone.0172727
M. Rasoulzadeh and H. Namazi, “Carboxymethyl cellulose/graphene oxide bio-nanocomposite hydrogel beads as anticancer drug carrier agent”, Carbohydr. Polym., vol. 168, 2017, 320-326, doi: 10.1016/j.carbpol.2017.03.014
S.M. Kuo, S.J. Chang, H.Y. Wang, S.C. Tang, and S.W. Yang, “Evaluation of the ability of xanthan gum/gellan gum/hyaluronan hydrogel membranes to prevent the adhesion of postrepaired tendons”, Carbohydr. Polym., vol. 114, 2014, 230-237, doi: 10.1016/j.carbpol.2014.07.049
R. Liu, S. Zhang, and X. Chen, “Injectable hydrogels for tendon and ligament tissue engineering”, J. Tissue Eng. Regen. Med., vol. 14, no. 9, 2016, 1333-1348, doi: 10.1002/term.3078
J. Li, E. Weber, S. Guth‐Gundel, M. Schuleit, A. Kuttler, C. Halleux, D.J. Mooney, “Tough composite hydrogels with high loading and local release of biological drugs”, Adv. Healthc. Mater., vol. 7, no. 9, 2018, 1701393, doi: 10.1002/adhm.201701393
W. Nafo, Hydrogel Biomaterials for Drug Delivery: Mechanisms, Design, and Drugs. Hydrogels - From Tradition to Innovative Platforms with Multiple Applications, 2022, doi: 10.5772/intechopen.103156
B.R. Freedman, A. Kuttler, N. Beckmann, S. Nam, D. Kent, M. Schuleit, D.J. Mooney, “Enhanced tendon healing by a tough hydrogel with an adhesive side and high drug-loading capacity”, Nat. Biomed. Eng, vol. 6, no. 10, 2022, 1167-1179, doi: 10.1038/s41551-021-00810-0
A. Pourjavadi and M. Doroudian, “Synthesis and characterization of semi-conductive nanocomposite based on hydrolyzed collagen and in vitro electrically controlled drug release study”, Polymer, vol. 76, 2015, 287-294, doi: 10.1016/j.polymer.2015.06.050
Y. Zheng, Y. Cheng, J. Chen, J. Ding, M. Li, C. Li, X. Chen, “Injectable hydrogel–microsphere construct with sequential degradation for locally synergistic chemotherapy”, ACS Appl. Mater. Interfaces, vol. 9, no. 4, 2017, 3487-3496, doi: 10.1021/acsami.6b15245
D. Docheva, S.A. Müller, M. Majewski, C. H. Evans,“Biologics for tendon repair”, Adv. Drug. Deliv. Rev., no. 84, 2015, 222-39, doi: 0.1016/j.addr.2014.11.015
S. Farnebo, L. Farnebo, M. Kim, C. Woon, H. Pham, J. Chang, “Optimized repopulation of tendon hydrogel: synergistic effects of growth factor combinations and adipose-derived stem cells”, Hand, vol. 12, no. 1, 2017, 68-77, doi: 10.1177/1558944715628005
W.R. Webb, T.P. Dale, A.J. Lomas, G. Zeng, I. Wimpenny, A.J. El Haj, G.Q. Chen “The application of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) scaffolds for tendon repair in the rat model”, Biomaterials, vol. 34, no. 28, 2013, 6683-6694, doi: 10.1016/j.biomaterials.2013.05.041
Q.Q. Yang, L. Zhang, F. Ju, Y.L. Zhou, “Sustained-release hydrogel-based rhynchophylline delivery system improved injured tendon repair”, Colloids Surf. B, vol. 205, 2021, 111876, doi: 10.1016/j.colsurfb.2021.111876
X. Xu, Y. Zhang, P. Ha, Y. Chen, C. Li, E. Yen, Z. Zheng, “A novel injectable fibromodulin‐releasing granular hydrogel for tendon healing and functional recovery”, Bioeng. Transl. Med., vol.8, no.1, 2023, e10355, doi: 10.1002/btm2.10355
A. Chattopadhyay, M.G. Galvez, M. Bachmann, A. Legrand, R. McGoldrick, A. Lovell, J. Chang, “Tendon Regeneration with Tendon Hydrogel-Based Cell Delivery: A Comparison of Fibroblasts and Adipose-Derived Stem Cells”, Plast. Reconst. Surg., vol. 138, no. 3, 2016, 617-626, doi: 10.1097/PRS.0000000000002515
Z. Yan, H. Yin, M. Nerlich, C.G. Pfeifer, D. Docheva, “Boosting tendon repair: interplay of cells, growth factors and scaffold-free and gel-based carriers” J. Exp. Orthop., vol. 5, no.1, 2018, 1-13, doi: 10.1186/s40634-017-0117-1
J. Chang, C. Hung Pham, S. Woon, A. Farnebo Legrand, Injectable composition for in-situ repair and regeneration of an injured ligament or tendon and methods of use No. US2018/0133369A1. United States: Patent Application Publication, 2017.
Y. Zhu, A. Xie, M. Li, C. Zhang and T. Ni, “Noninvasive Photochemical Sealing for Achilles Tendon Rupture by Combination of Upconversion Nanoparticles and Photochemical Tissue Bonding Technology”, BioMed Res. Int., vol. 2020, 2020, doi: 10.1155/2020/1753152
S. Farnebo, C.Y. Woon, T. Schmitt, L.M. Joubert, M. Kim, H. Pham, J. Chang, “Design and characterization of an injectable tendon hydrogel: a novel scaffold for guided tissue regeneration in the musculoskeletal system”, Tissue Eng. Part A, vol. 20, 9-10, 2014, 1550-1561, doi: 10.1089/ten.tea.2013.0207
S.D. Dutta, D.K. Patel, and K.T. Lim, “Functional cellulose-based hydrogels as extracellular matrices for tissue engineering”, J. Biol. Eng., vol. 13, no. 1, 2019, 1-19, doi: 10.1186/s13036-019-0177-0
Z. Bao, C. Xian, Q. Yuan, G. Liu, G, J. Wu, “Natural polymer‐based hydrogels with enhanced mechanical performances: preparation, structure, and property”, Adv. Healthc. Mater., vol.8, no.17, 2019, 1900670, doi: 10.1002/adhm.201900670
D.S. Kim, J.H. Kim, S.W. Baek, J.K. Lee, S.Y. Park, B. Choi, D.K. Han, “Controlled vitamin D delivery with injectable hyaluronic acid-based hydrogel for restoration of tendinopathy”, J. Tissue Eng., vol.13,2022, 20417314221122089, doi: 10.1177/20417314221122089
Descargas
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2023 Revista Mexicana de Ingenieria Biomedica

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial 4.0.
Una vez que el artículo es aceptado para su publicación en la RMIB, se les solicitará al autor principal o de correspondencia que revisen y firman las cartas de cesión de derechos correspondientes para llevar a cabo la autorización para la publicación del artículo. En dicho documento se autoriza a la RMIB a publicar, en cualquier medio sin limitaciones y sin ningún costo. Los autores pueden reutilizar partes del artículo en otros documentos y reproducir parte o la totalidad para su uso personal siempre que se haga referencia bibliográfica al RMIB. No obstante, todo tipo de publicación fuera de las publicaciones académicas del autor correspondiente o para otro tipo de trabajos derivados y publicados necesitaran de un permiso escrito de la RMIB.











